
Efficacy
EVKEEZA® cut LDL-C in half at 24 weeks in combo with low-fat diet and other LDL-C lowering therapies1,2
- Through week 24
- Through week 48
EVKEEZA reduced LDL-C levels by ~50% when added to current standard therapies1-3 (-3.4 mmol/L absolute change; LS mean treatment difference; 95% CI: -65% to -33%; n=65; P<0.0001)
LDL-C reductions were maintained through the open-label treatment period to week 481*
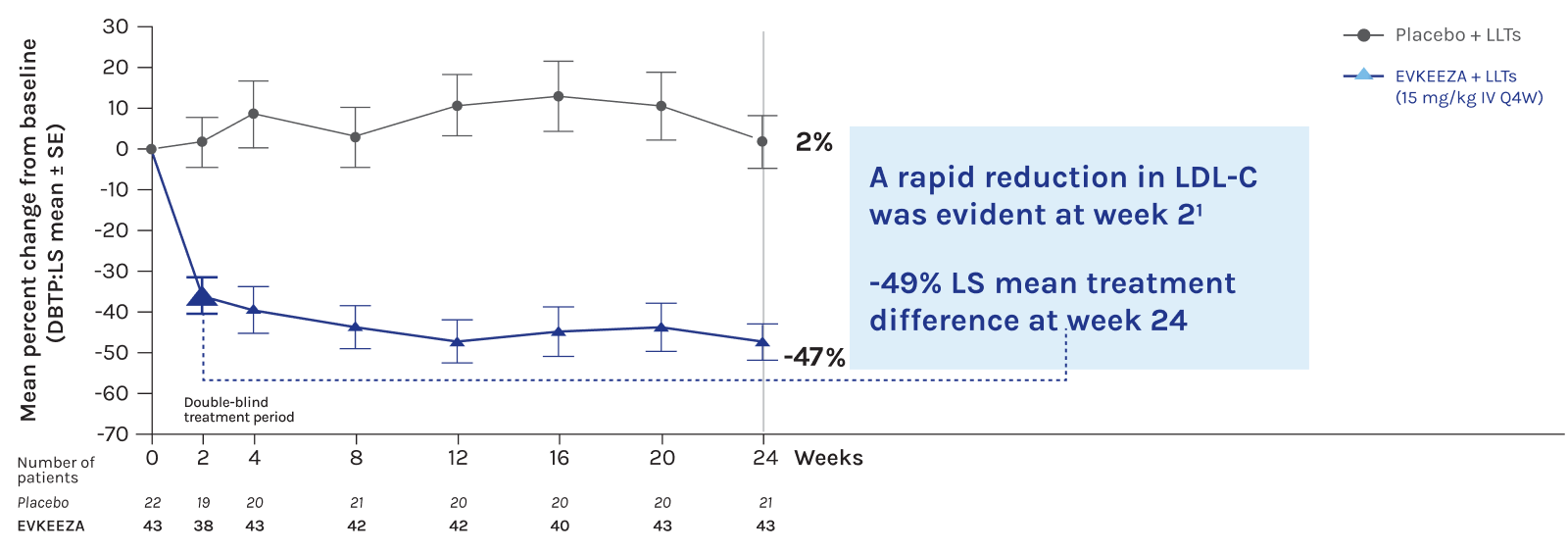
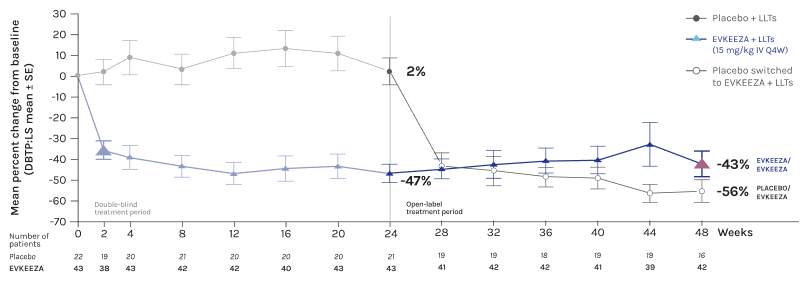
*After the double-blind treatment period, 64 of the 65 randomized patients who entered the open-label treatment period received evinacumab. The mean percent change in LDL-C from baseline to week 48 ranged from -42.7% to -55.8%.1
Adapted from Raal et al., 2020 and 2023.
LDL-C reductions were similar regardless of background therapy2
| Background therapy at baseline | No background therapy at baseline | |||
|---|---|---|---|---|
| Background therapy at baseline, mean (SD) | EVKEEZA 15 mg/kg IV Q4W, % (SD) | Placebo IV Q4W, % (SD) | EVKEEZA 15 mg/kg IV Q4W, % (SD) | Placebo IV Q4W, % (SD) |
| Statin | -47% (31), N=61 | 2% (32), N=61 | -46% (11), N=4 | -6% (23), N=4 |
| Ezetimibe | -53% (21), N=49 | -2% (31), N=49 | -28% (46), N=16 | 12% (34), N=16 |
| Lomitapide | -50% (23), N=14 | -17% (48), N=14 | -46% (32), N=51 | 5% (28), N=51 |
| PCSK9 inhibitor | -50% (32), N=50 | 2% (30), N=50 | -39% (20), N=15 | 1% (36), N=15 |
| Apheresis | -46% (18), N=22 | -7% (34), N=22 | -48% (34), N=43 | 7% (29), N=43 |
Adapted from Raal et al., 2020.
70% of patients taking EVKEEZA received a background of 3 LLTs during the clinical trial2
The proportion of patients in the placebo trial who were on at least three LLTs was 50%
ELIPSE-HoFH:
Phase 3, multicentre, double-blind, randomized, parallel-group, placebo-controlled study in patients 12 years and older with HoFH. Patients were taking a background of lipid-lowering therapies, including maximally tolerated statins, ezetimibe, PCSK9 inhibitor antibodies, lomitapide and lipoprotein apheresis. In the double-blind treatment period, 43 patients were randomized to receive EVKEEZA 15 mg/kg IV every 4 weeks and 22 patients to receive placebo. In the open-label treatment period, 64 patients received EVKEEZA 15 mg/kg IV every 4 weeks.1
Safety Profile
EVKEEZA has a demonstrated safety profile
Hypersensitivity, including anaphylaxis and infusion reaction (pruritus) have been reported. If occurs, discontinue treatment and treat according to standard of care.
Women of childbearing potential should use effective contraception during treatment and for at least 5 months after the last dose of EVKEEZA.
| Adverse Drug Reactions in HoFH Patients Treated with EVKEEZA in Placebo-Controlled Trials1 | |||
|---|---|---|---|
| System order class | Preferred term |
All EVKEEZA IV Doses* n = 117 (%) |
Placebo IV Q4W n = 54 (%) |
| Gastrointestinal disorders | Nausea | 5 | 1.9% |
| Abdominal pain | 3 | 1.9% | |
| Constipation | 3 | 0% | |
| General disorders and administration site conditions | Influenza like illness | 8 | 5.6% |
| Asthenia | 3 | 0% | |
| Infusion site pruritus | 2 | 0% | |
| Immune system disorders | Anaphylaxis | 1 | 0% |
| Infections and infestations | Nasopharyngitis | 14 | 13% |
| Upper respiratory tract infection | 3 | 0% | |
| Musculoskeletal and connective tissue disorders | Back pain | 5 | 3.7% |
| Pain in extremity | 5 | 0% | |
| Nervous system disorders | Dizziness | 6 | 0% |
| Respiratory, thoracic and mediastinal disorders | Rhinorrhoea | 3 | 0% |
*All EVKEEZA intravenous doses include 5 mg/kg IV Q4W data from Study R1500-CL-1643 plus 15 mg/kg IV Q4W from the double-blind treatment period of both Study R1500-CL-1643 and Study R1500-CL-1629.
LDL-C=low-density lipoprotein cholesterol; DBTP=double-blind treatment period; LS=least squares; SE=standard error; LLT=lipid-lowering therapy; IV=intravenous; Q4W=every 4 weeks; HoFH=homozygous familial hypercholesterolemia; PCSK9=proprotein convertase subtilisin/kexin type 9.
References:
- EVKEEZA Product Monograph. Ultragenyx Pharmaceutical, Inc. 2023.
- Raal FJ, Rosenson RS, Reeskamp LF, et al. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med. 2020;383(8):711-720.
- Wiegman A, et al. Evinacumab for pediatric patients with homozygous familial hypercholesterolemia. Circulation. 2024;149(5):343-353.
For children aged 5 to 11, EVKEEZA reduced LDL-C levels by ~50% when added to current standard therapies1,3 (-3.42 mmol/L absolute change; n=14; 95% CI: -68.8% to -27.8%)
Current standard therapies included:
- Statins
- Ezetimibe
- PCSK9 inhibitors*
- Lomitapide
- Apheresis
* PCSK9 inhibitor were permitted as part of the study however, no patients were on it at the time of the study
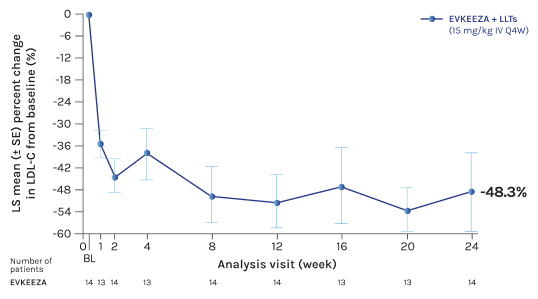
Adapted from Wiegman et al., 2024
* Primary endpoint; raw data shown as there was no missing data at the primary efficacy timepoint (24 weeks).
The LDL-C lowering effect was similar regardless of apheresis use or LDLR status4
Study R1500-CL-17100 was a Phase 1b/3 multicentre, three-part, single-arm, open-label study evaluating the efficacy, safety and tolerability of EVKEEZA in pediatric patients (≥ 5 to 11 years of age) with HoFH. The study includes three parts: Part A was a single-dose, open-label study to assess the safety, pharmacokinetics and pharmacodynamics of EVKEEZA 15 mg/kg IV in 6 patients with HoFH followed by a 16-week observational period to determine the dose for the rest of the study. Part B was a single-arm, 24-week, open-label treatment period evaluating the efficacy and safety of EVKEEZA 15 mg/kg IV every 4 weeks in 14 patients with HoFH. Part C is an extension study from Part A and Part B evaluating the long-term safety of EVKEEZA 15 mg/kg IV every 4 weeks in 20 patients with HoFH. It consists of a 48-week treatment period and a 24-week follow-up period (ongoing). Patients in Part C entered directly from Part A or Part B.

Safety Profile
EVKEEZA has a demonstrated safety profile in children aged 5-111,4
The safety profile of EVKEEZA observed in these patients was consistent with the safety profile observed in adults and adolescent patients aged 12 years and older, with the additional adverse reaction of fatigue. Fatigue was reported in 3 (15%) pediatric patients.
| Adverse Reactions | n (%) |
|---|---|
| Oropharyngeal pain | 3 (21.4) |
| Fatigue | 3 (15) |
| Upper abdominal pain | 2 (14.3) |
| Diarrhea | 2 (14.3) |
| Headache | 2 (14.3) |
| Nasopharyngitis | 2 (14.3) |
One patient experienced a general allergic event characterized by rash and contact dermatitis that was mild and did not lead to treatment discontinuation; there were no events involving hepatic disorders.
LDL-C=low-density lipoprotein cholesterol; PCSK9=proprotein convertase subtilisin/kexin type 9; HoFH=homozygous familial hypercholesterolemia; IV=intravenous; LS=least squares; SE=standard error; LLT=lipid-lowering therapy; Q4W=every 4 weeks; LDLR=lowdensity lipoprotein receptor; BL=baseline; CI=confidence interval.
References:
- EVKEEZA Product Monograph, Ultragenyx Pharmaceutical Inc. 2023.
- Raal FJ, Rosenson RS, Reeskamp LF, et al. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med. 2020;383(8):711-720.
- Raal, F, Rosenson, R, Reeskamp, L. et al. The Long-Term Efficacy and Safety of Evinacumab in Patients With Homozygous Familial Hypercholesterolemia. JACC Adv. 2023 Nov, 2 (9)
- Adam RC, Mintah IJ, Alexa-Braun CA, et al. Angiopoietin-like protein 3 governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. J Lipid Res. 2020;61(9):1271-1286.
- Wiegman A, et al. Evinacumab for pediatric patients with homozygous familial hypercholesterolemia. Circulation. 2024;149(5):343-353.



